#Moderna ships mRNA vaccine against novel coronavirus (mRNA-1273) for phase 1 study. #TheWallStreetJournal
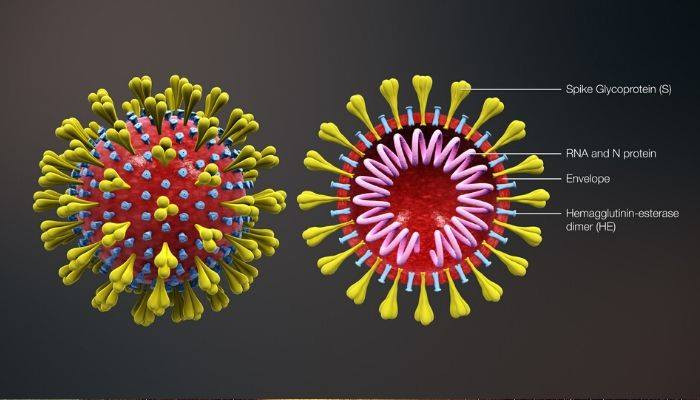 Moderna, Inc., (Nasdaq: MRNA) a clinical stage biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines to create a new generation of transformative medicines for patients today announced that it has released the first batch of mRNA-1273, the Company’s vaccine against the novel coronavirus, for human use. Vials of mRNA-1273 have been shipped to the National Institute of Allergy and Infectious Diseases (NIAID), a part of the National Institutes of Health (NIH) to be used in the planned Phase 1 study in the U.S. mRNA-1273 is an mRNA vaccine against the novel coronavirus encoding for a prefusion stabilized form of the Spike (S) protein, which was selected by Moderna in collaboration with investigators at the NIAID Vaccine Research Center (VRC). Manufacture of this batch was funded by the Coalition for Epidemic Preparedness Innovations (CEPI). mRNA-1273 is an mRNA vaccine against the novel coronavirus encoding for a prefusion stabilized form of the Spike (S) protein, which was designed by Moderna in collaboration with NIAID. The S protein complex is necessary for membrane fusion and host cell infection and has been the target of vaccines against the coronaviruses responsible for Middle Eastern Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). |

Woman who suffered flesh-eating disease after insect bite receives a face transplant
83504.02.2026, 00:07
India rushes to contain deadly Nipah virus outbreak after five cases confirmed
91124.01.2026, 20:43
Thousands of NYC nurses strike for better staffing and pay (video)
85912.01.2026, 23:38
Nestle issues global recall of some baby formula products over toxin fears
121807.01.2026, 20:43
Air pollution India's biggest health crisis since Covid, warn doctors
133126.12.2025, 17:41
"What Is This Mushroom?"... Japanese Man Trusts AI, Ends Up in Emergency Room
141527.11.2025, 22:53
