CanSino's #COVID_19 #vaccine candidate approved for military use in China
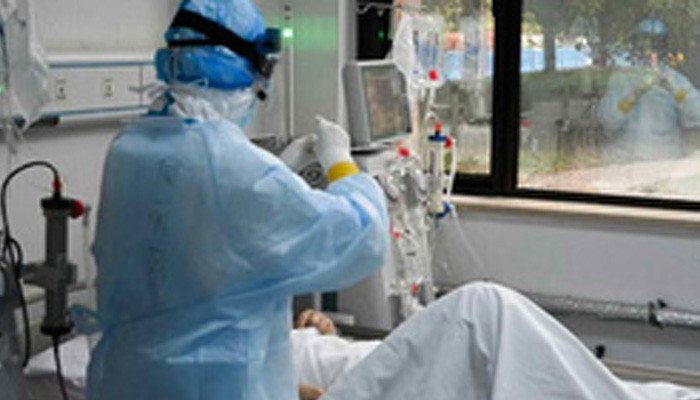 China's military has received the greenlight to use a COVID-19 vaccine candidate developed by its research unit and CanSino Biologics after clinical trials proved it was safe and somewhat efficient, the company said on Monday. The Ad5-nCoV is one of the eight vaccine candidates being developed by Chinese companies and researchers approved to be moved into human trials for the respiratory disease caused by the new coronavirus. The shot also won approval for human testing in Canada. China’s Central Military Commission approved the use of the vaccine by the military on June 25 for a period of one year, CanSino said in a filing. The vaccine candidate was developed jointly by CanSino and the Beijing Institute of Biotechnology in the Academy of Military Medical Sciences. “The Ad5-nCoV is currently limited to military use only and its use cannot be expanded to a broader vaccination range without the approval of the Logistics Support Department,” CanSino said, referring to the Central Military Commission department where the military use of the vaccine was approved. |

Archaeology Team Uncovers Major Ancient Settlement Site on University Grounds (photo)
55426.02.2026, 09:13
NASA to Provide Coverage of Artemis II Wet Dress Rehearsal
59724.02.2026, 00:54
Alien files incoming: Trump orders government release of UFO records
80720.02.2026, 12:49
Single-dose HIV vaccine candidate induces neutralizing antibodies
75803.02.2026, 19:19
Apple ‘runs on Anthropic,’ says Mark Gurman
79201.02.2026, 23:53
Meet the Kennewick Man: Face of 'most important' ancient American revealed after 8,500 years
81925.01.2026, 17:07
